Ampcera® Argyrodite Li6PS5Cl0.5Br0.5 Sulfide Solid Electrolyte, Fine Powder (D50 ~ 5 um)
SKU: PO0184
Ampcera® Argyrodite Li6PS5Cl0.5Br0.5 Sulfide Solid Electrolyte, Fine Powder (D50 ~ 5 um)
If you need more than 1kg, please contact Ampcera Inc. (info@ampcera.com) directly for bulk pricing.
Manufacturer: Ampcera Inc.
Product Number: PO0184
Composition: : Li6PS5Cl0.5Br0.5
Material Type: Argyrodite, Li-argyrodite crystalline phase
Purity: synthesized from >99.9% precursor materials
Product Form: White powder
Particle Size: Pass 325 mesh, D50 ~ 5 µm. This fine powder can be directly used to make composite solid electrolytes or to mix with cathode materials as solid state catholyte. The finer powder helps to improve the cathode-electrolyte interface contact.
Ionic Conductivity: ~2.8 mS/cm at room temperature
Electronic Conductivity: ≤10-8 S/cm at room temperature
Applications: Solid state electrolyte material for all solid-state lithium-ion batteries. Cathode electrolyte (catholyte).
Storage and Cautions: Water sensitive. Store and operate in a dry environment.
About Argyrodite solid electrolyte:
Argyrodites, Li6PS5X (X = Cl, Br), are considered to be among the most promising solid-state electrolytes for solid-state batteries. Argyrodite-type crystalline materials, such as Li6PS5Cl0.5Br0.5, are promising solid electrolytes for all-solid-state lithium-ion batteries because of their high ionic conductivity (up to > 3.5 mS/cm at room temperature), good processability and excellent electrochemical stability. Li6PS5Cl0.5Br0.5 has been reported to exhibit higher ionic conductivity than Li6PS5Cl and Li6PS5Br. With its proprietary technology, Ampcera Inc. is the first company in the world that has successfully commercialized the Argyrodite-type Li6PS5Cl0.5Br0.5 solid electrolyte material with a production capacity of more than one metric ton per year. Please contact us for bulk order discount. Customized processing is also available to meet the technical specifications requested by customers.
* All the solid-state electrolyte materials sold by MSE Supplies are under the trademark of Ampcera.
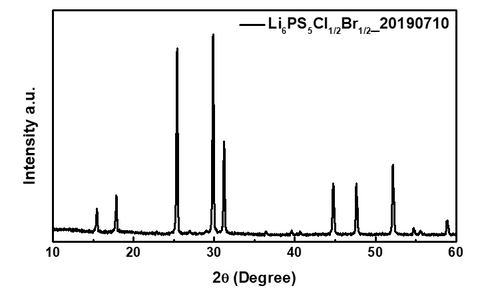
X-Ray Diffraction Spectrum of Li6PS5Cl0.5Br0.5, which shows an Argyrodite phase (F-43m) structure

- Sylvain Boulineau, Matthieu Courty, Jean-Marie Tarascon, Virginie Viallet, Mechanochemical synthesis of Li-argyrodite Li6PS5X (X = Cl, Br, I) as sulfur-based solid electrolytes for all solid state batteries application, Solid State Ionics, Volume 221, 3 August 2012, Pages 1-5 https://doi.org/10.1016/j.ssi.2012.06.008
-
Interface Stability of Argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in Bulk All-Solid-State Batteries
Jérémie Auvergniot, Alice Cassel, Jean-Bernard Ledeuil, Virginie Viallet, Vincent Seznec, and Rémi Dedryvère, Chemistry of Materials 2017 29 (9), 3883-3890, DOI: 10.1021/acs.chemmater.6b04990 - Zhi Deng, Yifei Mo and Shyue Ping Ong, Computational studies of solid-state alkali conduction in rechargeable alkali-ion batteries, NPG Asia Materials (2016) 8, e254; doi:10.1038/am.2016.7
- Yu, C., Ganapathy, S., Hageman, J., van Eijck, L., van Eck, E., Zhang, L., Wagemaker, M. (2018). Facile Synthesis toward the Optimal Structure-Conductivity Characteristics of the Argyrodite Li6PS5Cl Solid-State Electrolyte. ACS applied materials & interfaces, 10(39), 3329633306. doi:10.1021/acsami.8b07476
- Yuki Kato et al. High-power all-solid-state batteries using sulfide superionic conductors, Nature Energy (2016). download pdf from the above link
- R. P. Rao, S. Adams, Studies of lithium argyrodite solid electrolytes for all‐solid‐state batteries, physica status solidi (a) – applications and materials science, Volume 208, Issue 8, August 2011, Pages 1804-1807
- Heng Wang, Chuang Yu, Swapna Ganapathy, Ernst R.H. van Eck, Lambert van Eijck and Marnix Wagemaker, A lithium argyrodite Li6PS5Cl0.5Br0.5 electrolyte with improved bulk and interfacial conductivity, Journal of Power Sources, 10.1016/j.jpowsour.2018.11.029, 412, (29-36), (2019).
- Qing Zhang, Daxian Cao, Yi Ma, Avi Natan, Peter Aurora and Hongli Zhu, Sulfide‐Based Solid‐State Electrolytes: Synthesis, Stability, and Potential for All‐Solid‐State Batteries, Advanced Materials, 31, 44, (2019).
- Parvin Adeli, J. David Bazak, Kern Ho Park, Ivan Kochetkov, Ashfia Huq, Gillian R. Goward and Linda F. Nazar, Boosting Solid‐State Diffusivity and Conductivity in Lithium Superionic Argyrodites by Halide Substitution, Angewandte Chemie, 131, 26, (8773-8778), (2019).



