Human ApoE (Apolipoprotein E) CLIA Kit
SKU: E-CL-H0383
Human ApoE (Apolipoprotein E) CLIA Kit
| Detection Range | 4.69-300 ng/mL |
| Sensitivity | 2.81 ng/mL |
Product Details
Properties
| Assay type | Sandwich-CLIA |
| Format | 96T/48T |
| Assay time | 3.5h |
| Detection range | 4.69-300 ng/mL |
| Sensitivity | 2.81 ng/mL |
| Sample type &Sample volume | serum, plasma and other biological fluids; 100μL |
| Specificity | This kit recognizes Human ApoE in samples. No significant cross-reactivity or interference between Human ApoE and analogues was observed. |
| Reproducibility | Both intra-CV and inter-CV are < 15%. |
| Application | This CLIA kit applies to the in vitro quantitative determination of Human ApoE concentrations in serum, plasma and other biological fluids. |
Test Principle
Kit components & Storage
An unopened kit can be stored at 2-8℃ for 1 month. If the kit is not supposed to be used within 1 month, store the components separately according to the following conditions once the kit is received.
| Item | Specifications | Storage |
|---|---|---|
| Micro CLIA Plate(Dismountable) | 96T: 8 wells ×12 strips 48T: 8 wells ×6 strips |
-20℃, 6 months |
| Reference Standard | 96T: 2 vials 48T: 1 vial |
|
| Concentrated Biotinylated Detection Ab (100×) | 96T: 1 vial, 120 μL 48T: 1 vial, 60 μL |
|
| Concentrated HRP Conjugate (100×) | 96T: 1 vial, 120 μL 48T: 1 vial, 60 μL |
-20℃(Protect from light), 6 months |
| Reference Standard & Sample Diluent | 1 vial, 20 mL | 2-8°C, 6 months |
| Biotinylated Detection Ab Diluent | 1 vial, 14 mL | |
| HRP Conjugate Diluent | 1 vial, 14 mL | |
| Concentrated Wash Buffer (25×) | 1 vial, 30 mL | |
| Substrate Reagent A | 1 vial, 5 mL | 2-8℃(Protect from light) |
| Substrate Reagent B | 1 vial, 5 mL | |
| Plate Sealer | 5 pieces | |
| Manual | 1 copy | |
| Certificate of Analysis | 1 copy |
Technical Data:
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, mid range and high level Human ApoE were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, mid range and high level Human ApoE were tested on 3 different plates, 20 replicates in each plate.
| Intra-assay Precision | Inter-assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (ng/mL) | 15.52 | 32.03 | 108.28 | 14.29 | 32.46 | 98.89 |
| Standard deviation | 1.64 | 3.52 | 10.82 | 1.66 | 2.50 | 11.30 |
| CV (%) | 10.57 | 10.99 | 9.99 | 11.62 | 7.70 | 11.43 |
Recovery
The recovery of Human ApoE spiked at three different levels in samples throughout the range of the assay was evaluated in various matrices.
| Sample Type | Range (%) | Average Recovery (%) |
|---|---|---|
| Serum(n=8) | 87-100 | 92 |
| EDTA plasma(n=8) | 96-111 | 104 |
| Cell culture media(n=8) | 99-114 | 106 |
Target Information
Assay Procedures
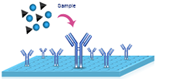 |
1.Add 100 μL of standard or sample to each well. Incubate for 90 min at 37℃. |
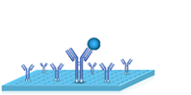 |

2.Remove the liquid. |
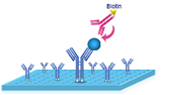 |

3.Add 100 μL Biotinylated Detection Ab. Incubate 1 hour at 37℃. Aspirate and wash 3 times. |
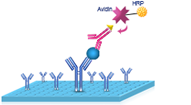 |

4.Add 100 μL HRP Conjugate. Incubate 30 min at 37℃. Aspirate and wash 5 times. |
 |

5.Add 100 μL Substrate Mixture Solution. Incubate for 5 min at 37℃. |
 |

6.Fluorescence appeared. Measure the RLU value with the Chemiluminescence immunoassay analyzer. Calculation of the results. |



