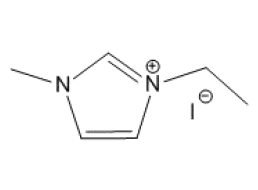
MSE PRO 1-ethyl-3-methylimidazolium Iodide (C6H11N2I) , >99%
SKU: CM1045
MSE PRO™ 1-ethyl-3-methylimidazolium Iodide (C6H11N2I), >99%
1-ethyl-3-methylimidazolium iodide (C6H11N2I) is an ionic liquid produced from methylimidazole and iodoethane. Mixing it and 1-ethyl-3-methylimidazolium tetrafluoroborate (EMImBF4) increases capacitance, and thus can be used in electric double-layer capacitors (EDLCs). Additionally, it combines with aluminum chloride to form 1-ethyl-3-methylimidazolium halogenoaluminate ionic liquid, which is an effective solvent for Friedel–Crafts acylation of ferrocene and OLED materials.Specification
| Item | Value |
| Product Name | 1-ethyl-3-methylimidazolium Iodide |
| Chemical Formula | C6H11N2I |
| Synonym(s) |
|
| SKU# | CM1045, 100g |
| CAS# | 35935-34-3 |
| Molecular Weight | 238.07 g/mol |
| Appearance | White or light yellow solid |
| Purity | >99% |
| Condition to Avoid | Light Sensitive, Hygroscopic |
| Store Under Inert Gas | Yes |
| Melting Point | 79 °C |
| Solubility in Water | Soluble |
References
[1] Electrochemical characteristics pyrolytic graphite| mixture of 1-ethyl-3-methylimidazolium tetrafluoroborate and 1-ethyl-3-methylimidazolium iodide interface. Journal of Electroanalytical Chemistry 719 (2014): 133-137.
