QuicKey Human MAU(Microalbuminuria) ELISA Kit
SKU: E-TSEL-H0005
QuicKey Human MAU(Microalbuminuria) ELISA Kit
| Detection Range | 1.56-100 ng/mL |
| Sensitivity | 0.30 ng/mL |
Product Details
Properties
| Assay type | Sandwich-ELISA |
| Format | 96T |
| Assay time | 2.5h |
| Detection range | 1.56-100 ng/mL |
| Sensitivity | 0.30 ng/mL |
| Sample type &Sample volume | urine; 50μL |
| Specificity | This kit recognizes Human MAU in samples. No significant or interference between Human MAU and analogues was observed. |
| Reproducibility | Both intra-CV and inter-CV are < 10%. |
| Application | This ELISA kit applies to the in vitro quantitative determination of Human MAU concentrations in urine.Please consult technical support for the applicability if other biological fluids need to be tested. |
Test Principle
This ELISA kit uses the Sandwich-ELISA principle. The micro ELISA plate provided in this kit has been pre-coated with an antibody specific to Human MAU. Samples (or Standards) and biotinylated detection antibody specific for Human MAU are added to the micro ELISA plate wells. Human MAU would combine with the specific antibody. Then Avidin-Horseradish Peroxidase (HRP) conjugate are added successively to each micro plate well and incubated. Free components are washed away. The substrate solution is added to each well. Only those wells that contain Human MAU, biotinylated detection antibody and Avidin-HRP conjugate will appear blue in color. The enzyme-substrate reaction is terminated by the addition of stop solution and the color turns yellow. The optical density (OD) is measured spectrophotometrically at a wavelength of 450 ± 2 nm. The OD value is proportional to the concentration of Human MAU. You can calculate the concentration of Human MAU in the samples by comparing the OD of the samples to the standard curve.
Kit Components and Storage
An unopened kit can be stored at 2-8℃ for six months. After test, the unused wells and reagents should be stored according to the table below.
| Item | Specifications | Storage conditions after test |
|---|---|---|
| Micro ELISA Plate (Dismountable) | 96T: 8 wells ×12 strips 48T: 8 wells ×6 strips |
2-8℃, 1 month |
| Reference Standard | 96T: 2 vials 48T: 1 vial |
Discard unused reconstituted standard and dilutions |
| Reference Standard & Sample Diluent | 1 vial, 20 mL | 2-8℃ |
| Biotinylated Detection Ab Working Solution | 1 vial, 6 mL | |
| HRP Conjugate Diluent | 1 vial, 14 mL | |
| Concentrated Wash Buffer (25×) | 1 vial, 30 mL | |
| Concentrated HRP Conjugate (100×) | 96T: 1 vial, 120 μL 48T: 1 vial, 60 μL |
2-8℃ (Protect from light) |
| Substrate Reagent | 1 vial, 10 mL | |
| Stop Solution | 1 vial, 10 mL | 2-8℃ |
| Plate Sealer | 5 pieces | |
| Product Description | 1 copy | |
| Certificate of Analysis | 1 copy |
Technical Data:
Precision
Intra-assay Precision (Precision within an assay): 3 samples with low, mid range and high level Human MAU were tested 20 times on one plate, respectively.
Inter-assay Precision (Precision between assays): 3 samples with low, mid range and high level Human MAU were tested on 3 different plates, 20 replicates in each plate.
| Intra-assay Precision | Inter-assay Precision | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 1 | 2 | 3 |
| n | 20 | 20 | 20 | 20 | 20 | 20 |
| Mean (ng/mL) | 4.14 | 12.39 | 49.50 | 4.74 | 11.35 | 40.46 |
| Standard deviation | 0.22 | 0.56 | 2.66 | 0.24 | 0.56 | 2.04 |
| CV (%) | 5.31 | 4.52 | 5.37 | 5.06 | 4.93 | 5.04 |
Recovery
The recovery of Human MAU spiked at three different levels in samples throughout the range of the assay was evaluated in various matrices.
| Sample Type | Range (%) | Average Recovery (%) |
|---|---|---|
| Urine(n=8) | 97-104 | 101 |
Target Information
Background
1. Glassock R J. Prevention of Microalbuminuria in Type 2 Diabetes: Millimeters or Milligrams? [J]. Journal of the American Society of Nephrology Jasn, 2006, 17(12):3276.
2. Deckert T, Feldtrasmussen B, Borchjohnsen K, et al. Albuminuria reflects widespread vascular damage [J]. Diabetologia, 1989, 32(4):219.
| Database Links | SwissProt: P02768 Entrez Gene: 213 |
| Research Area | Cancer, Cardiovascular, Metabolism, Developmental biology, Signal transduction, Stem cells |
Assay Procedures
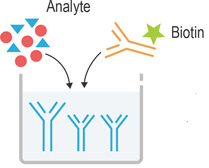 |
1. Add 50μL standard or sample to the wells, immediately add 50μL Biotinylated Detection Ab working solution to each well. Incubate for 90 min at 37°C |
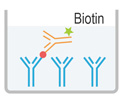 |
2. Aspirate and wash the plate for 3 times |
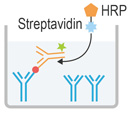 |
3. Add 100μL HRP conjugate working solution. Incubate for 30 min at 37°C. Aspirate and wash the plate for 5 times |
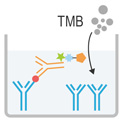 |
4. Add 90μL Substrate Reagent. Incubate for 15 min at 37°C |
 |
5. Add 50μL Stop Solution |
 |
6. Read the plate at 450nm immediately. Calculation of the results |



