Ascorbate Peroxidase (APX) Activity Assay Kit
SKU: E-BC-K353-S-100
Ascorbate Peroxidase (APX) Activity Assay Kit
| SKU # | E-BC-K353-S |
| Detection Instrument | Spectrophotometer (290 nm) |
| Detection method | Colorimetric method |
Product Details
Properties
| Synonyms | APX |
| Sample type | Plant tissue |
| Sensitivity | 0.071 U/g tissue |
| Detection range | 0.071-47 U/g tissue |
| Detection Method | Colorimetric method |
| Assay type | Enzyme Activity |
| Assay time | 60 min |
| Precision | Average inter-assay CV: 6.400% | Average intra-assay CV: 4.800% |
| Other instruments required | Vortex mixer, Micropipettor, Water bath, Incubator, Centrifuge |
| Other reagents required | Normal saline (0.9% NaCl), PBS (0.01 M, pH 7.4) |
| Storage | 2-8℃ |
| Valid period | 12 months |
Images
H Y Lee et al investigate the effects of melatonin in high light stress tolerance in Arabidopsis thaliana. Ascorbate peroxidase (APX) activity of Arabidopsis leaf was determined using APX activity assay kit (E-BC-K353-S).
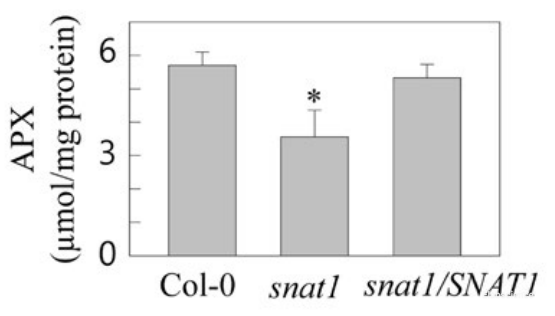
In high light stress situation, the APX activity was significantly reduced in snat1 mutant group. (P<0.05)
Dilution of Sample
It is recommended to take 2~3 samples with expected large difference to do pre-experiment before formal experiment and dilute the sample according to the result of the pre-experiment and the detection range (0.071-47 U/g tissue).
The recommended dilution factor for different samples is as follows (for reference only):
| Sample type | Dilution factor |
| 10% Epipremnum aureum tissue homogenization | 1 |
| 10% Carrot tissue homogenization | 1 |
| 10% Green pepper tissue homogenization | 1 |
| 10% Mushrooms tissue homogenization | 1 |
Note: The diluent is normal saline (0.9% NaCl) or PBS (0.01 M, pH 7.4).
Detection Principle
Ascorbate Peroxidase (APX) can catalyze the reaction between ascorbic acid (ASA) and hydrogen peroxide (H2O2), and ASA can be oxidized to monodehydroascorbic acid (MDASA). The absorbance of solution at 290 nm will decline as the oxidation of ASA. The APX activity can be calculated by detecting the decrease of A290.
Kit Components & Storage
| Item | Component |
Size 1 (50 assays) |
Size 2 (100 assays) |
Storage |
| Reagent 1 | Extracting Solution | 60 mL × 1 vial | 60 mL × 2 vials | 2-8°C, 12 months |
| Reagent 2 | Buffer Solution | 40 mL × 1 vial | 40 mL ×2 vials | 2-8°C, 12 months |
| Reagent 3 | Substrate | Powder ×1 vial | Powder ×2 vials | 2-8°C, 12 months shading light |
| Reagent 4 | Substrate Solution | 12 mL ×1 vial | 12 mL ×1 vial | 2-8°C, 12 months |
Note: The reagents must be stored strictly according to the preservation conditions in the above table. The reagents in different kits cannot be mixed with each other. For a small volume of reagents, please centrifuge before use, so as not to obtain sufficient amount of reagents.
Technical Data:
Parameter:
Intra-assay Precision
Three human serum samples were assayed in replicates of 20 to determine precision within an assay. (CV = Coefficient of Variation)
| Parameters | Sample 1 | Sample 2 | Sample 3 |
| Mean (U/g tissue) | 1.50 | 18.40 | 32.80 |
| %CV | 5.2 | 4.7 | 4.5 |
Inter-assay Precision
Three human serum samples were assayed 17 times in duplicate by three operators to determine precision between assays.
| Parameters | Sample 1 | Sample 2 | Sample 3 |
| Mean (U/g tissue) | 1.50 | 18.40 | 32.80 |
| %CV | 5.7 | 6.7 | 6.8 |
Recovery
Take three samples of high concentration, middle concentration and low concentration to test the samples of each concentration for 6 times parallelly to get the average recovery rate of 96%.
| Standard 1 | Standard 2 | Standard 3 | |
| Expected Conc. (U/g tissue) | 8.5 | 26.5 | 40.5 |
| Observed Conc. (U/g tissue) | 8.4 | 24.4 | 39.3 |
| Recovery rate (%) | 99 | 92 | 97 |
Sensitivity
The analytical sensitivity of the assay is 0.071 U/g tissue. This was determined by adding two standard deviations to the mean O.D. obtained when the zero standard was assayed 20 times, and calculating the corresponding concentration.



